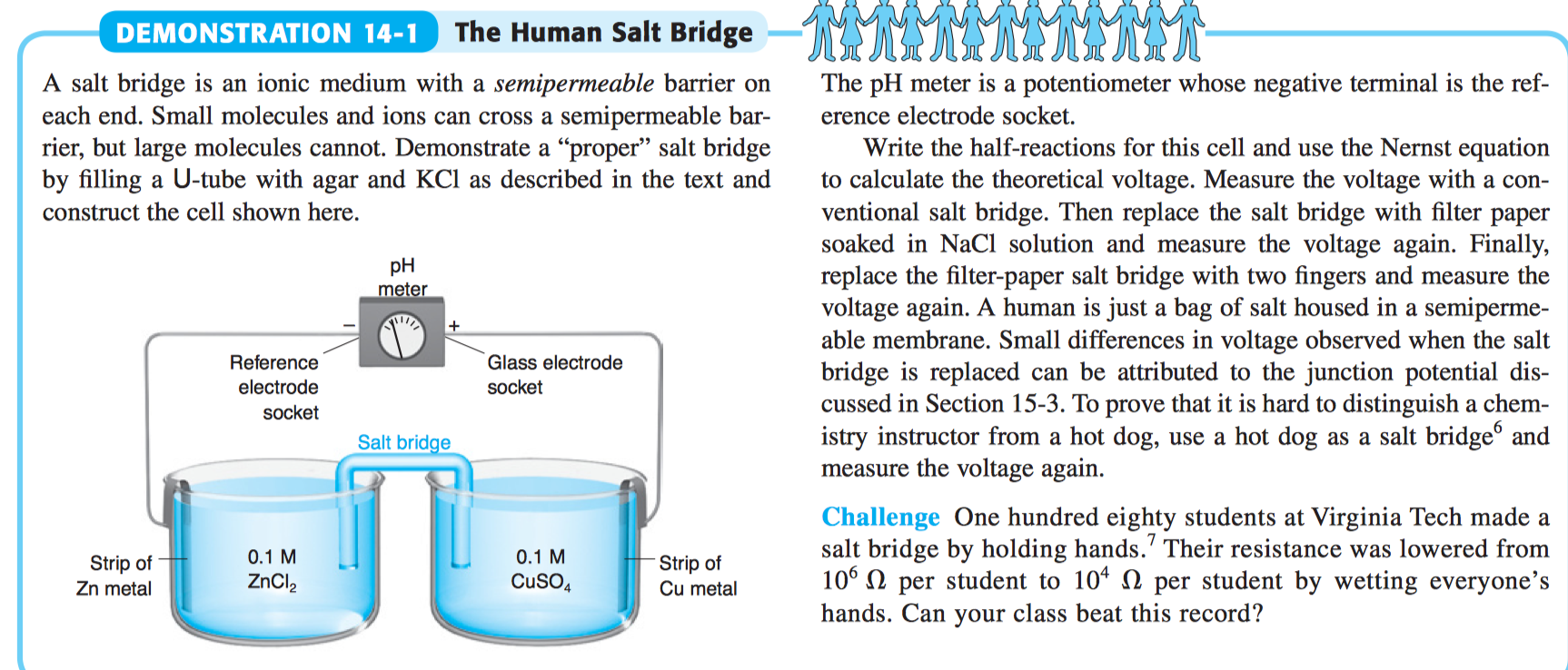
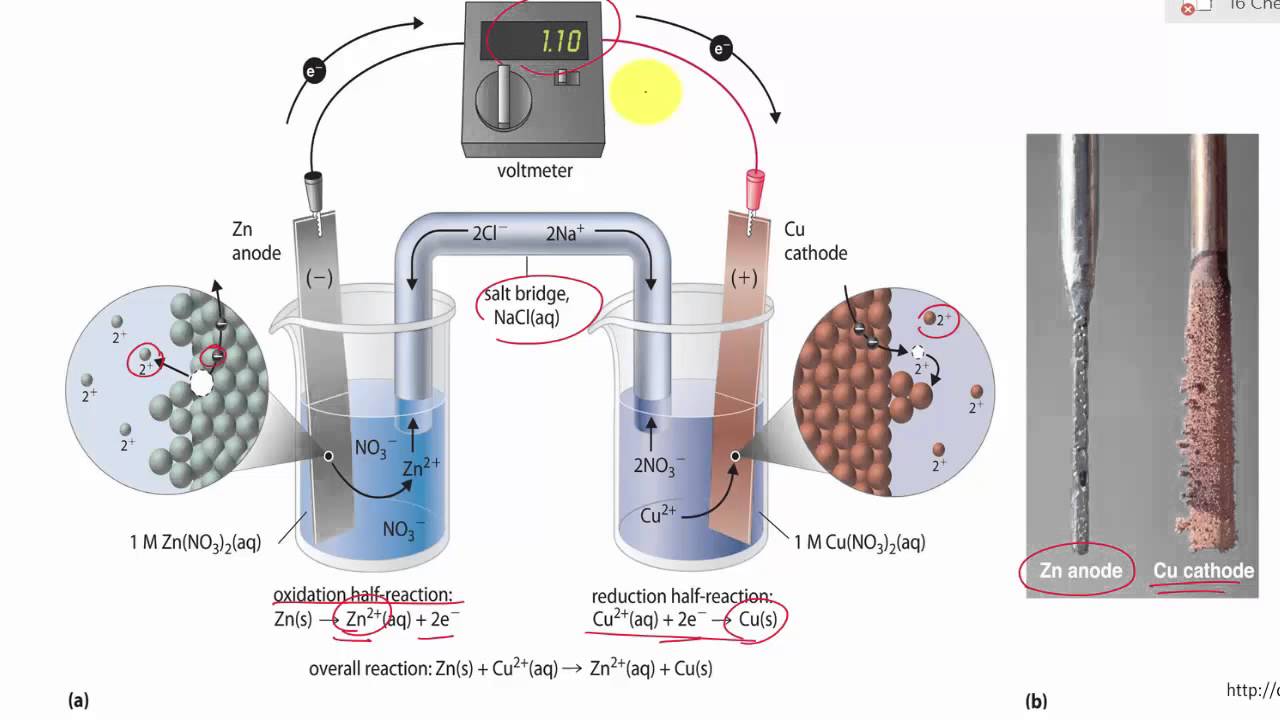
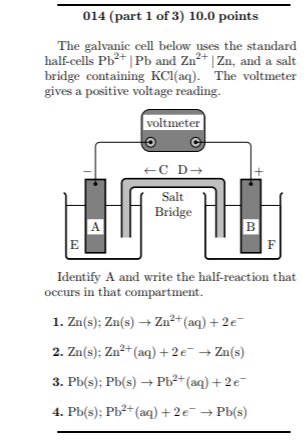
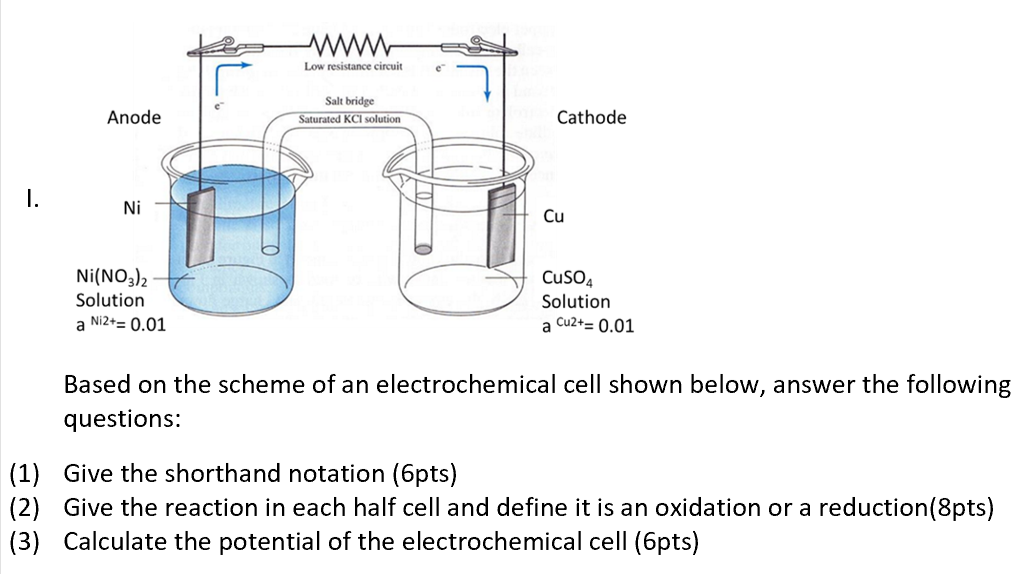
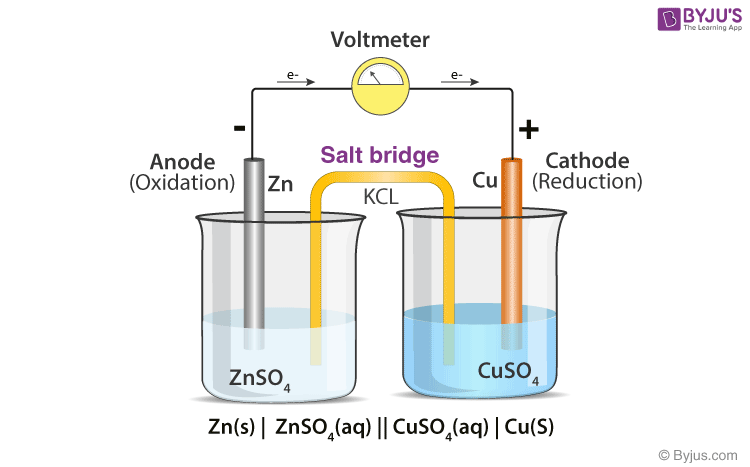
A) Schematic of the micro-agar salt bridge. Three percent agarose in 3... | Download Scientific Diagram
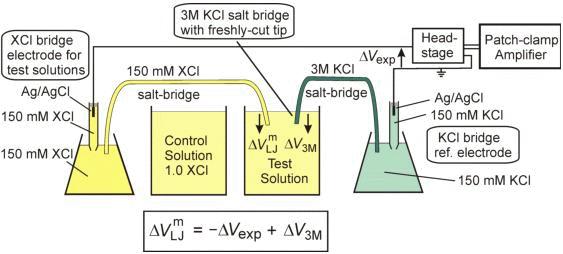
An optimised 3 M KCl salt-bridge technique used to measure and validate theoretical liquid junction potential values in patch-cl

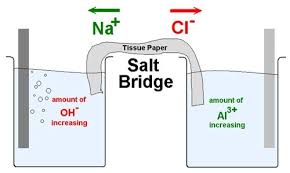
Statement I: KCl, NaCl, NH4Cl, etc., cannot be used in the salt bridge of a cell containing silver.Statement II : A salt bridge contains concentrated solution of an inert electrolyte like KCl,

KCl cannot be used as a salt bridge for the cell Cu(s) abs(CuSO(4)(aq))abs(AgNO(3)(aq)) Ag(s) because:

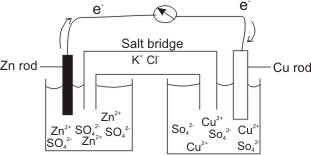
physical chemistry - Why is it important to use a salt bridge in a voltaic cell? Can a wire be used? - Chemistry Stack Exchange
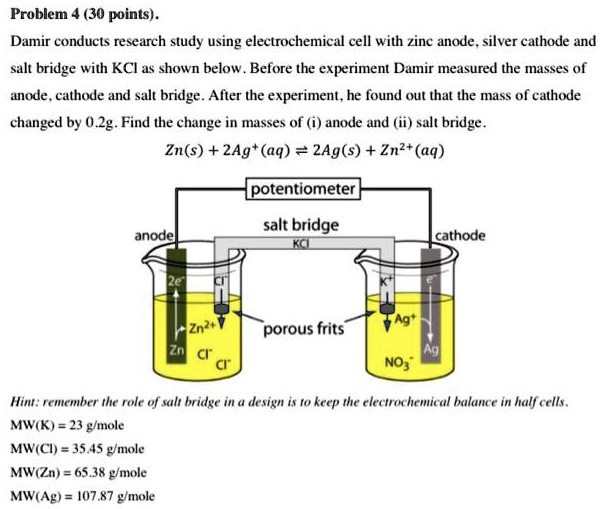
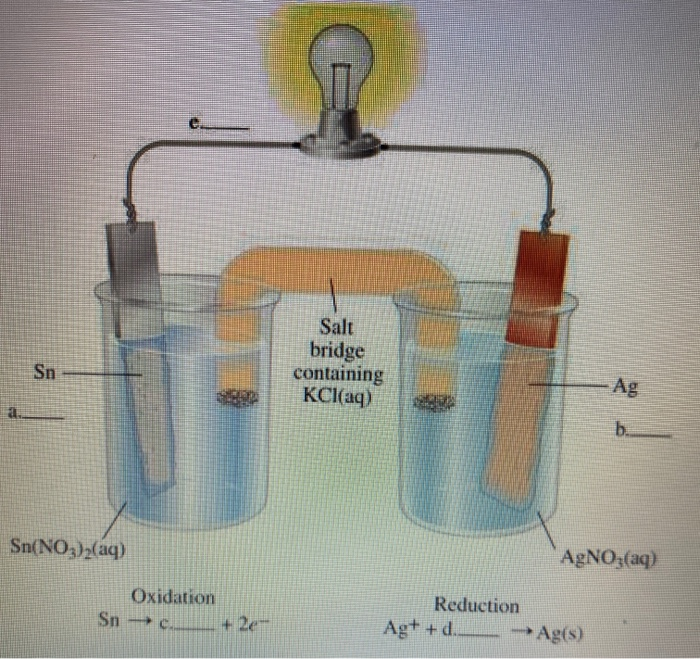
SOLVED: Problem 4 (30 points) . Damir conducts research study using electrochemical cell with zine anode silver cathode und salt bridge with KCl as shown below. Before the experiment Damir measured the